Cell free DNA Prenatal Screening
Prenatal screening today is testing for diseases or conditions in a fetus before it is born and estimating the maternal risk to develop pregnancy-related disorders
As recommended by the Fetal Medicine Foundation UK and expert clinicians, prenatal screening includes two main steps:
Step 1
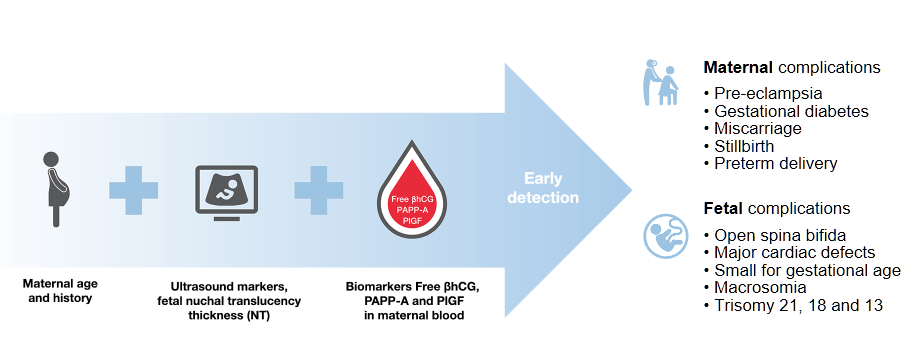
Population-wide first trimester combined screening with maternal characteristics, ultrasound markers and maternal serum markers.
First trimester combined testing shows a high performance for detection of trisomies: 90% for trisomy 21, 97% for trisomy 18 and 92% for trisomy 13 at a false positive rate of 4%Ref-1.
The benefit of combined testing is an early detection of a wide range of pregnancy complications, including pre-eclampsia
With one single maternal blood draw and one test on B·R·A·H·M·S KRYPTOR compact PLUS to measure free βhCG, PAPP-A and PlGF concentrations, it is possible to determine the risks for fetal trisomies, pre-eclampsia as well as other adverse outcome pregnancy complications.
Step 2
Cell free DNA (cfDNA) prenatal screening is based upon evaluation of the proportion of placental cfDNA in the blood of pregnant women, thereby estimating the risks for fetal trisomies and other genetic disorders.
cfDNA testing or so-called NIPT (non invasive prenatal screening test) is an advanced screening test for fetal trisomies. Analysis of cfDNA in maternal blood in singleton pregnancies could detect more than 99% of fetuses with trisomy 21, 98% of fetuses with trisomy 18 and 99% of fetuses with trisomy 13 at a combined false positive rate of 0,13%Ref-2.
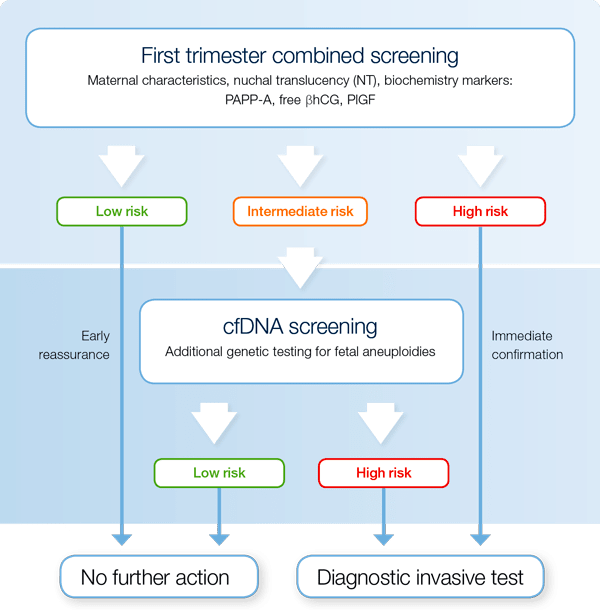
The contingent screening strategy
Contingent screening strategy provides the answer to the question "How should cfDNA prenatal screening be integrated in routine prenatal screening practices?"Ref-3
The contingent screening strategy combines routine first trimester screening for several pregnancy complications with additional NIPT or cell free DNA screening as a second step for an intermediate risk group of fetal trisomies.
 |
 |
Find here a 10 minute animation about contingent cfDNA prenatal screening.
- Contingent screening predicts a wide range of pregnancy complications
- Contingent screening reduces the number of unnecessary invasive tests
To learn more about the Fetal Medicine Foundation UK approach to clinical implementation of cfDNA prenatal test, please watch Dr. Gil's presentation during the 9th International Workshop on Prenatal Screening held in Berlin in 2017.
Reasons why first trimester combined screening cannot be substituted by cfDNA prenatal screening
- First trimester combined screening comprises much more than screening for trisomies because it predicts a range of fetal and maternal complicationsRef-4;
- First trimester maternal serum screening includes measurement of PAPP-A, free βhCG and optionally PlGF and AFP can be performed in the same sample and on the same platform. For this reason it is beneficial in screening for pre-eclampsia, fetal growth restriction and preterm birthRef-5,6,7;
- cfDNA screening has an average of 3% failure rate which is often related to the low fetal fraction. Fetal fraction is reported to be low in conditions with decreased placental volume such as in trisomies 18 and 13Ref-8;
- First trimester ultrasound can detect 49% of major structural anomalies, including skeletal and cardiac defects in fetusRef-9;
- About 10% of atypical chromosomal abnormalities are associated with an adverse outcome that cannot be identified by common NIPT programsRef-10;
- For fetal fraction below 10% it would be preferable to use the risk estimate from the first-line method of screening as the prior risk and modify this by the appropriate positive or negative likelihood ratio for a given fetal fraction from the cfDNA testRef-11.
Further links on prenatal screening products and assays
- Download our brochure about cell free DNA prenatal screening.
- Download our literature review about contingent screening.
- Find here a 10 minute animation about contingent cfDNA prenatal screening.
- Find here all our prenatal screening brochures, product sheets and other informational material.
References
Ref-1: M. Santorum, D. Wright, A. Syngelaki, N. Karagioti, and K. H. Nicolaides, “Accuracy of first-trimester combined test in screening for trisomies 21, 18 and 13.,” Ultrasound Obstet. Gynecol., vol. 49, no. 6, pp. 714–720, Jun. 2017.
Ref-2: M. M. Gil, V. Accurti, B. Santacruz, M. N. Plana, and K. H. Nicolaides, “Analysis of cell-free DNA in maternal blood in screening for aneuploidies: updated meta-analysis.,” Ultrasound Obstet. Gynecol., vol. 50, no. 3, pp. 302–314, Sep. 2017.
Ref-3: K. H. Nicolaides, A. Syngelaki, L. C. Poon, M. M. Gil, and D. Wright, “First-trimester contingent screening for trisomies 21, 18 and 13 by biomarkers and maternal blood cell-free DNA testing.,” Fetal Diagn. Ther., vol. 35, no. 3, pp. 185–92, 2014.
Ref-4: K. H. Nicolaides, “Turning the pyramid of prenatal care,” Fetal Diagn. Ther., vol. 29, no. 3, pp. 183–196, 2011.
Ref-5: R. Akolekar, A. Syngelaki, L. Poon, D. Wright, and K. H. Nicolaides, “Competing risks model in early screening for preeclampsia by biophysical and biochemical markers.,” Fetal Diagn. Ther., vol. 33, no. 1, pp. 8–15, 2013.
Ref-6: L. C. Y. Poon, A. Syngelaki, R. Akolekar, J. Lai, and K. H. Nicolaides, “Combined screening for preeclampsia and small for gestational age at 11-13 weeks.,” Fetal Diagn. Ther., vol. 33, no. 1, pp. 16–27, 2013.
Ref-7: J. Beta, F. E. Bredaki, J. Rodriguez Calvo, R. Akolekar, and K. H. Nicolaides, “Maternal serum α-fetoprotein at 11-13 weeks’ gestation in spontaneous early preterm delivery,” Fetal Diagn. Ther., vol. 30, no. 2, pp. 88–93, 2011.
Ref-8: R. Revello, L. Sarno, A. Ispas, R. Akolekar, and K. H. Nicolaides, “Screening for trisomies by cell-free DNA testing of maternal blood: consequences of a failed result.,” Ultrasound Obstet. Gynecol., vol. 47, no. 6, pp. 698–704, Jun. 2016.
Ref-9: M. Grande, M. Arigita, V. Borobio, J. M. Jimenez, S. Fernandez, and A. Borrell, “First-trimester detection of structural abnormalities and the role of aneuploidy markers.,” Ultrasound Obstet. Gynecol., vol. 39, no. 2, pp. 157–63, Feb. 2012.
Ref-10: K. O. Kagan, M. Hoopmann, R. Hammer, R. Stressig, and P. Kozlowski, “Screening for chromosomal abnormalities by first trimester combined screening and noninvasive prenatal testing.,” Ultraschall Med., vol. 36, no. 1, pp. 40–6, Feb. 2015.
Ref-11: D. Wright, a Wright, and K. H. Nicolaides, “A unified approach to risk assessment for fetal aneuploidies.,” Ultrasound Obstet. Gynecol., vol. 45, no. 1, pp. 48–54, 2015.