COVID-19 and Scarce Hospital Resources:
B·R·A·H·M·S MR-proADM KRYPTOR for Risk Stratification to Support Decisions on the Required Level of Care Learn morePre-eclampsia: 3rd Trimester Screening
It's time to implement it: 3rd Trimester Pre-eclampsia Screening
Screening for late-onset pre-eclampsia using sFlt-1 and PlGF now available

Late onset pre-eclampsia represents 75% of all pre-eclampsia cases and is associated with significant maternal mortality and morbidity.
While screening and aspirin administration in the first trimester can help prevent early onset pre-eclampsia, there is still a pressing need for better methods to predict and prevent late onset cases.
Screening at week 35-36 using PlGF and sFlt-1 allows to detect late-onset pre-eclampsia, opening the door to a prevention with timed birth. Developed by the Fetal Medicine Foundation, this screening concept is now available with B·R·A·H·M·S PlGF plus KRYPTOR and B·R·A·H·M·S sFlt-1 KRYPTOR, offering healthcare professionals an important tool for identifying and managing at-risk pregnancies.
For further insights into this crucial topic, be sure to check out some of the most important publications in the field: Literature Review - 3rd Trimester Pre-eclampsia Screening (378 KB).
Video about Prediction of pre-eclampsia in 3rd trimester: Tune in to hear from the esteemed opinion leader, Professor Kypros Nicolaides, as he shares the latest scientific insights on predicting pre-eclampsia in the third trimester. Don't miss out on the opportunity to learn from this renowned expert in the field.
Do you want to learn more about the screening concept mentioned above, or speak with a sales representative to explore how it could benefit your practice or patients? We would love to hear from you! Please don't hesitate to contact us for further information and support.
Statistics Training
Prenatal Screening Webinars
Prediction of pre-eclampsia in 3rd trimester
Hear the latest scientific information on pre-eclampsia from renowned opinion leader Professor Kypros Nicolaides
We invite you to watch this video featuring Professor Kypros Nicolaides, a distinguished expert in the field. He present the latest scientific insights on predicting pre-eclampsia during the third trimester. To further enrich your understanding, the presentation is followed by a Q&A section where you can gain more knowledge from the expert. Don't miss out on this valuable learning opportunity!
Improved pre-eclampsia management with sFlt-1/PlGF ratio
Join us to learn from health care professionals who already introduced sFlt-1/PlGF in their clinical routine
Now available on demand
The live webinar is dedicated to the topic of improved diagnosis and prognosis of pre-eclampsia with the aid of sFlt-1/PlGF biomarker ratio. Join us to learn from health care professionals who already introduced sFlt-1/PlGF in their clinical routine. You will have an opportunity to address your questions to the clinical practitioners, exchange your ideas and learn about use of sFlt-1/PlGF on examples of clinical cases.
A Certificate of Attendance will be sent to participants.
Program
- Implementation of sFlt-1/PlGF ratio in clinical routine: A retrospective study
Martin Overgaard, Denmark - Evaluation of the prognostic value of the sFlt-1/PlGF ratio in early-onset pre-eclampsia
Paul Guerby, France - The aid of sFlt-1/PlGF ratio in pre-eclampsia patient management on examples of clinical cases
Ignacio Herraiz, Spain - Clinical value of pre-eclampsia biomarkers in high-risk pregnancies: Case reports
Roman Kapustin, Russia - Live Q&A
Don't miss our free on-demand webinars!
Register to watch the webinar recording
Let’s stay connected!
Continue receiving Thermo Fisher Scientific emails and content by clicking the "Subscribe now!" button below.
Self-determining Dilution Factor (SDDF)
Optimized lab workflow with self-determining dilution factor (SDDF)
Watch how self-determining dilution factor (SDDF) is calculating dilution factor and executes the dilution with no user interference.
Improved management of out of range (OOR) samples
Fast, accurate and reliable lab test results enable confident clinical decisions. Delays in reporting results can often have detrimental impact on both patient and healthcare provider, and as such, turnaround time is a key performance indicator of well-functioning laboratories. A frequent challenge to report results quickly, is the accurate measurement and processing of an out of range sample. By using BꞏRꞏAꞏHꞏMꞏS KRYPTOR Analyzers, which utilize Nobel Prize winning TRACE technology, it is now possible to eliminate unnecessary processing steps in out of range sample management and optimize turnaround time.
- Benefits of Self-determining dilution factor >
- The challenge in detail >
- Reducing turnaround time with BꞏRꞏAꞏHꞏMꞏS KRYPTOR Analyzers >
Benefits of Self-determining dilution factor
Benefits of Self-determining dilution factor are
- Reduce hands-on time
- Enhance turnaround time
- Save cost and wastage
- Avoid dilution errors
- Minimize hook effect and enhance accuracy
The challenge in detail
Processing an out of range sample would typically require the following steps:
Disadvantages of the above processing steps are
- An increased turnaround time
- Delayed patient results
- Increased error rates
Reducing turnaround time with BꞏRꞏAꞏHꞏMꞏS KRYPTOR Analyzers
BꞏRꞏAꞏHꞏMꞏS KRYPTOR Analyzers are the only immuno analyzers in the market that fully automated all necessary steps which are required for a dilution.
Using Nobel Prize winning TRACE (Time Resolved Amplified Cryptate Emission) technology, BꞏRꞏAꞏHꞏMꞏS KRYPTOR Analyzers are able to detect the OOR samples within few minutes into the incubation period and carry out the dilution without any user intervention. This unique feature which optimizes laboratory workflow is named “Self-determining dilution factor” (SDDF)
How does Self-determining dilution factor work?
The incubation period in B·R·A·H·M·S KRYPTOR Analyzers is divided in two phases.
- Detection phase
- Counting phase.
Incubation starts when the conjugates and sample are dispensed into a well, then the detection phase begins. During this phase by using TRACE, the kinetics of bound & unbound signal will be calculated and precisely predict if the sample concentration will be out of range at the end of incubation period. If sample concentration is predicted to be out of range, the self-determining dilution factor will determine the correct dilution factor autonomously and execute the dilution with no user intervention. Normally it takes BꞏRꞏAꞏHꞏMꞏS KRYPTOR Analyzers only one attempt to identify and perform the dilution with correct dilution factor.
Once the sample concentration is in the direct measuring range, counting phase will begin. At the end of counting phase, measurement is completed, and result will be directly delivered to the user.
Hospital Admission or Outpatient Treatment?
Benefit of Thermo Scientific B·R·A·H·M·S MR-proADM KRYPTOR in reducing hospital admission shown for the first time in a randomized control trial.
Two independent observational cohort studies have previously identified MR-proADM as the best risk stratification marker. The biomarker safely and accurately supported level of care decisions.Ref-1-2
The IDEAL study, a randomised controlled trial has confirmed observational data in real life patient careRef-3. Safe hospital discharge increased by 50% using the MR-proADM guided triage.
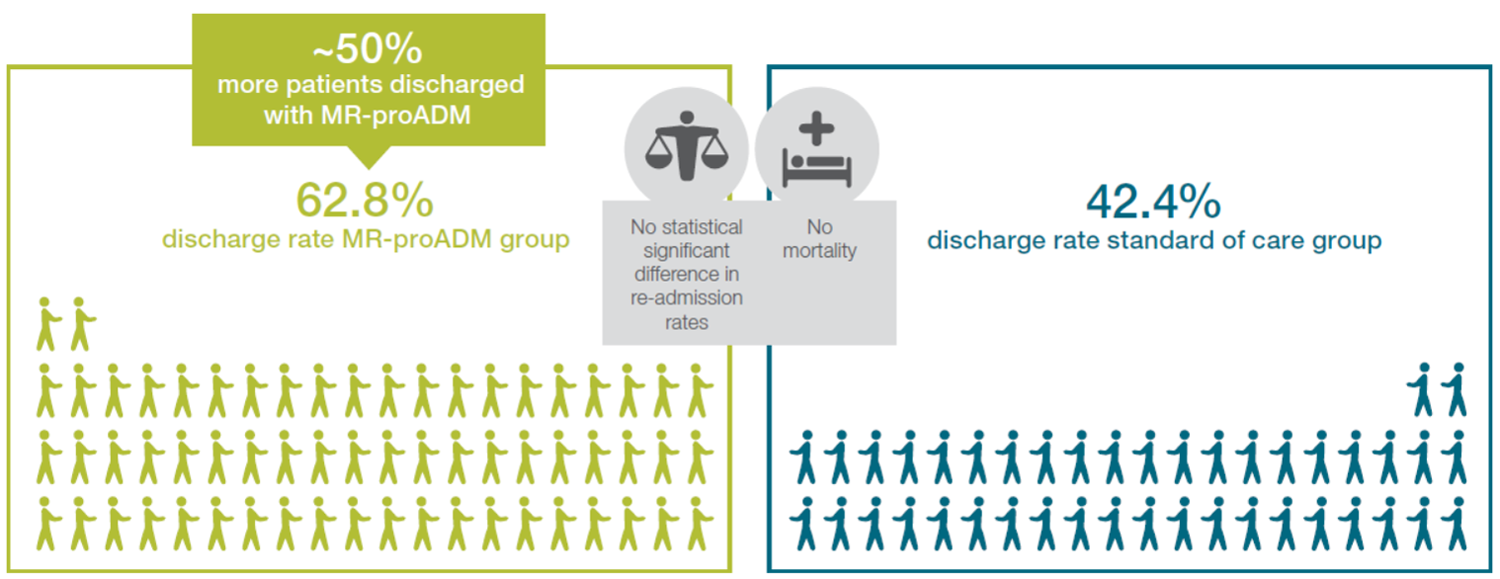 Figure 1: Number of patients (in %) admitted with signs of infection to the ED that could be discharged for outpatient treatment, based on either clinical assessment alone (standard of care, n=99) or a combination of clinical assessment and MR-proADM value (MR-proADM based, n=86).
Figure 1: Number of patients (in %) admitted with signs of infection to the ED that could be discharged for outpatient treatment, based on either clinical assessment alone (standard of care, n=99) or a combination of clinical assessment and MR-proADM value (MR-proADM based, n=86).
The algorithm of patients derivation is depicted in figure 2. Mortality: zero patients in in both groups. 14-days readmission: 3 patients (7.1%) in Standard of Care, 5 patients (9.3%) in MR-proADM based, p-value=1. 14 days representation rate: 8 patients (19.0%) in Standard of Care, 9 patients (16.7%) in MR-proADM based, p-value=0.973.
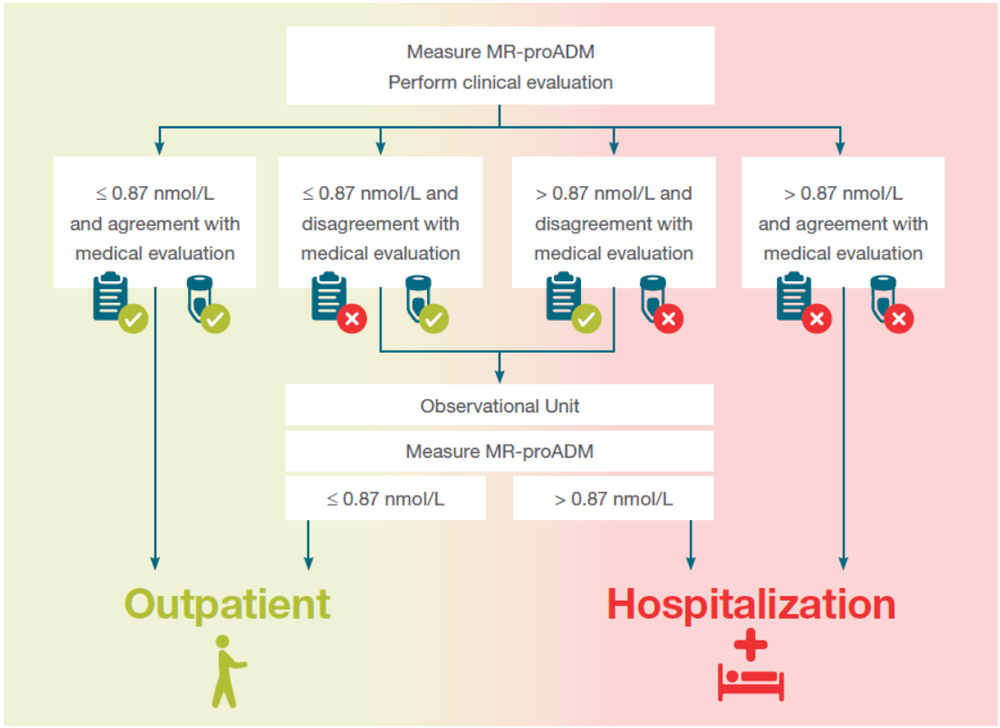
In patients with suspected infection presenting to the ED the MR-proADM algorithm can help to
- Identify patients at risk who require hospitalization (“red flag”)
- Rule-out severe conditions and increase safe discharge for up to 50% more patients compared to standard of care
- Optimize patient workflow
Presentation in the ED with suspicion of infection
Figure 2: MR-proADM guided algorithm for the triage of patients presenting to the ED with suspicion of infection as used in the IDEAL study
References hospital admission
Ref-1: Elke, G., et al., The use of mid-regional proadrenomedullin to identify disease severity and treatment response to sepsis – a secondary analysis of a large randomised controlled trial. Crit Care, 2018. 22(1): p. 79. doi: 10.1186/ s13054-018-2001-5.
Ref-2: Saeed, K., et al., The early identification of disease progression in patients with suspected infection presenting to the emergency department: a multi-centre derivation and validation study. Crit Care, 2019. 23(1): p. 40. doi: 10.1186/ s13054-019-2329-5.
Ref-3: Gonzalez del Castillo J. et al., Midregional proadrenomedullin (MR-proADM) algorithm reduces hospitalization rate by identifying low risk patients in the ED safely treatable as out-patients 2020 Poster P538 at eISICEM.